
silver bromide and silver iodide are insoluble even with the addition of ammonia. Less ionic silver chloride is soluble only after complexation with ammonia. Silver fluoride is quite ionic and soluble in water.

The example of silver halides may be considered in which there is polarizing cation and increasing polarizable anions. Solubility of salts in polar solvents like water is affected by polarization. An appreciable amount of polarization leads to intense absorption bands.
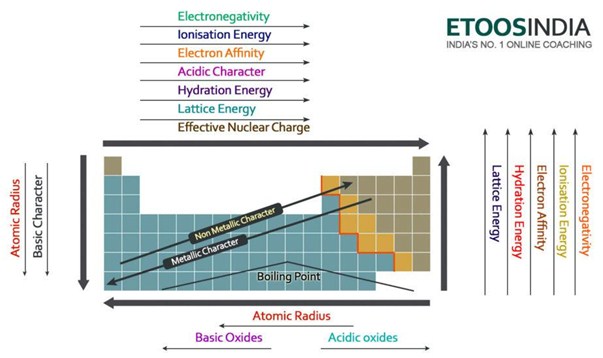
#Lattice energy trend periodic table compounds series#
(ii) In a series of halides of ions such as Ag +, the fluorides and chlorides are colourless ions is usually an indication of an appreciable amount of nonpolar character or some other unusual structural feature. With a few exceptions, the white metal sulphides are only those of alkali and alkaline earth metals. (i) The oxides of colourless cations are usually white but the corresponding sulphides are likely to be deeply coloured if the cation is one which has a tendency to polarize anions. Thus:Ĭolour deepening tendency polarization of anion size of anion The increase in nonpolar character of inorganic salts is manifested in the appearance or enhancement of colour. This explains almost identical chemical and physical properties of the above mentioned pairs of elements. Such elements will form bonds of a similar type in the corresponding compounds. If both moves are made simultaneously, as in a diagonal relationship then two elements of similar polarizing power may result, e.g., polarizing power of Be 2+ and Al 3+ is almost similar as their ionic potential ( ) are also almost similar. In a vertical group, with the increase power of the cation will correspondingly decrease. Consequently' the polarizing power will also increase. On moving to the right across a period in the periodic table the charge of the cation increases and the size decreases. The diagonal relationship observed between the following pairs of elements can also be explained with the help of Fajan's rules. We know that chemistry of lithium, berylium and boron resembles with that of magnesium, aluminium and silicon, respectively. The fourth Fajan's rule suggests that, in general, the non-transition elements are more ionic than the transition elements because their cations have lower polarizing power and so the cations are more stable. According to rules (2) and (3) the most stable anions are those which are small and have only a small charge. Similarly, the small halide ions will favour an ionic bond because they will form ions having the least polarizability. Thus the large alkali metal ions will prefer to form ionic bond. Rules(1), (3) and (4) indicate that the cations which are large and have small charge and possess inert gas electronic configuration should possess least polarizing power e.g., the large alkali metal ions. On the basis of these important general rules it becomes possible to predict the type of bond that a given element is likely to prefer. It is shown in table by the comparison of the melting points of anhydrous chlorides of IA and IB group of periodic table.Įffect of 8 and 18 electronic shell upon the covalent character Hence, if the charge and size are kept nearly constant, cations with 18-electron structure cause greater anion deformation than those with 8-electron arrangements.
Thus the cation should possess an electronic configuration which is not that of an inert gas. (4) Cation with non-inert gas atom structure _ The cations with the inert gas electron configuration are most effective in shielding the nuclear charge from its surface while the cations with non-inert gas atom structure have positive fields at their surfaces and consequently will possess high polarizing powers.


 0 kommentar(er)
0 kommentar(er)
